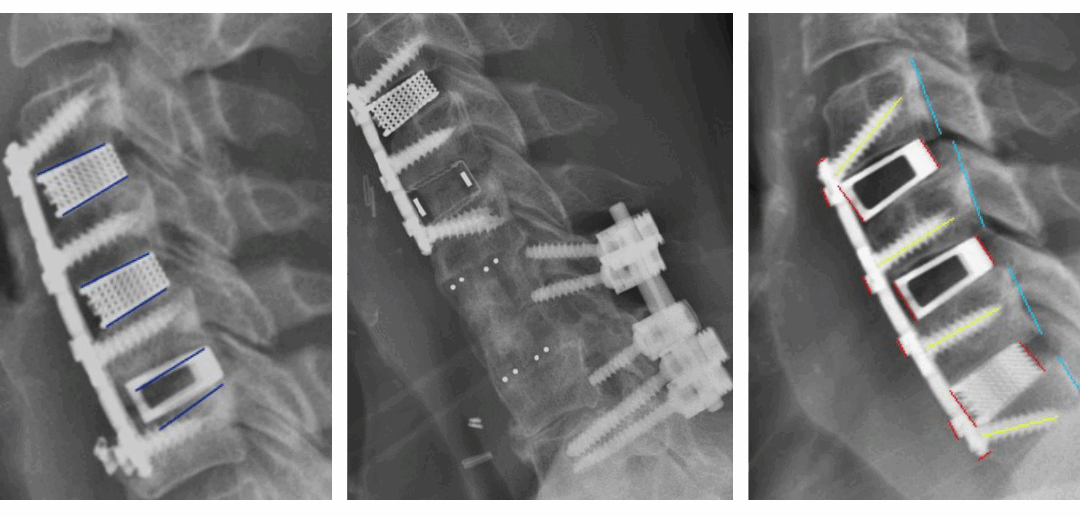
Nexus Spine is excited to share pilot study results of a comparative radiographic assessment of ACDF outcomes in a series of patients who were selected for common risk factors of subsidence, instability, and delayed bone growth. Specifically, the patients were over 66 years of age, had poor bone quality, and/or required cervical fusion surgery at three or more contiguous levels. Each patient received a Nexus Spine Tranquil® cervical interbody cage at one or more spinal levels, and, as a control, some patients received one or more competing interbody devices at adjacent level(s).
Post-operative radiographic assessment of the treated levels at each of two weeks, six weeks, six months, and twelve months revealed that the levels treated with Nexus Spine’s Tranquil® interbody devices had no measurable subsidence. In contrast, the levels treated with the other interbody devices demonstrated measurable subsidence as soon as two weeks in more than 66% of the levels treated. Subsidence is a primary indicator of spinal instability and pain. Accordingly, the results of this study suggest that Nexus Spine’s patented technology amounts to a significant improvement over the competing devices.

Another compelling result of the study is that each of the patients experienced total maintenance of restored disc height and sagittal alignment at all levels treated with Tranquil® cervical interbody cages. This is exceptional when compared to the levels treated with competitive cages, which experienced subsidence. Also, peer-reviewed publications demonstrate competitive devices that exhibit at least 3 mm of subsidence in more than 52% of patients. Nexus Spine’s Tranquil® interbody cages performed much better, with no subsidence.
This comparative study was independently designed and conducted by Dr. Peter Campbell, of Shreveport, LA, without funding, control nor input from Nexus Spine. Dr Campbell shared, “I wanted to compare outcomes of the seemingly best available technologies.” He continued with, “My radiographic outcomes made clear that Tranquil was the one device that ameliorated subsidence. Compared to other currently available devices on the market, Tranquil doesn’t subside.”
“If we can solve the key challenges for the highest-risk patients,” said David Hawkes, President of Nexus Spine, “then we can improve the universal standard of care.” David further explained, “Matching the stiffness of the host bone is key. This pilot study is very exciting because it demonstrates that our patented Tranquil® interbody implants are better at preventing subsidence. We are also looking at how much faster our devices provide stability. Subsidence and prolonged instability are both consequences of using an interbody that is too stiff.”
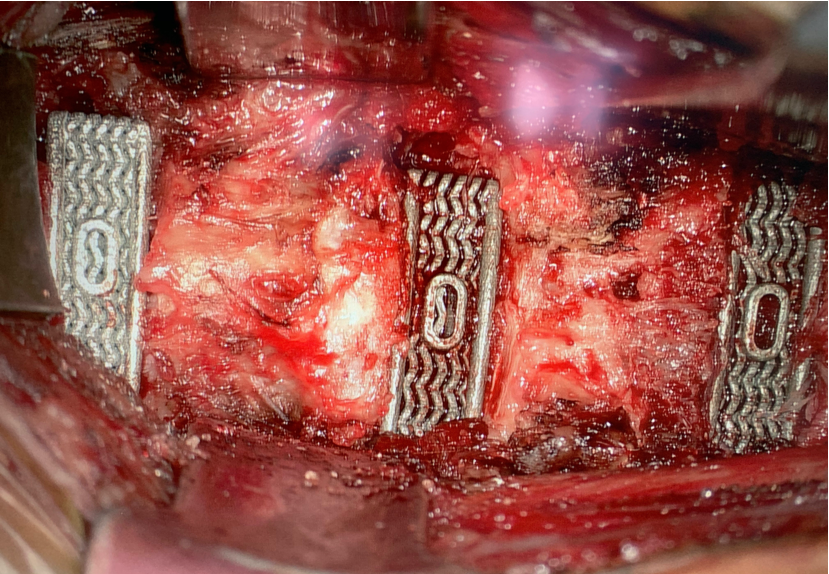
Nexus Spine believes the results from this pilot study provide encouraging data for patient outcomes. With such promising results, Nexus Spine plans to complete its review of the 64 patients in the cohort, including looking at the rate of achieving segmental stability.
Among its vast patent portfolio, Nexus Spine holds U.S. Patent No. 12,279,967, entitled “Porous Interbody Spacer,” which has a priority date that extends back to 2015. This patent relates to low-stiffness, porous spinal cages that are designed to mitigate subsidence by limiting contact stress at the bone-to-implant interface. Its broad claims provide strong protection of the proprietary technology enabling the outstanding results described above, thereby allowing Nexus Spine to take the lead in providing the interbody cages that exhibited the exceptional outcomes of the pilot study. This patent is available to view here.